Sif4 Lewis Structure Molecular Geometry
SiF4 (silicon tetrafluoride) has ane silicon cantlet and iv fluorine atoms.
In the lewis structure of SiF4, there are iv single bonds around the silicon atom, with 4 fluorine atoms attached to information technology, and on each fluorine atom, in that location are three lonely pairs.
Steps
Here'due south how you lot can draw the SiF4 lewis construction pace by step.
Step #1: draw sketch
Stride #2: mark lone pairs
Step #three: marker charges (if there are)
Permit's break down each step in detail.
#1 Draw Sketch
- Showtime, determine the total number of valence electrons
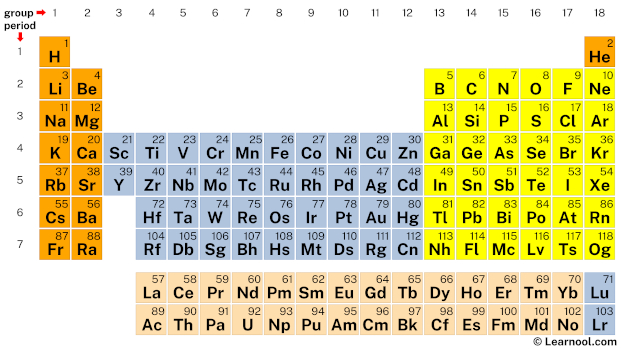
In the periodic table, silicon lies in grouping 14, and fluorine lies in group 17.
Hence, silicon has 4 valence electrons and fluorine has seven valence electrons.
Since SiF4 has one silicon atom and four fluorine atoms, and then…
Valence electrons of one silicon atom = iv × 1 = iv
Valence electrons of four fluorine atoms = seven × 4 = 28
And the total valence electrons = four + 28 = 32
Learn how to find: Silicon Valence Electrons and Fluorine Valence Electrons
- Second, find the total electron pairs
Nosotros have a total of 32 valence electrons. And when nosotros divide this value by two, we get the value of total electron pairs.
Full electron pairs = full valence electrons ÷ two
So the total electron pairs = 32 ÷ 2 = xvi
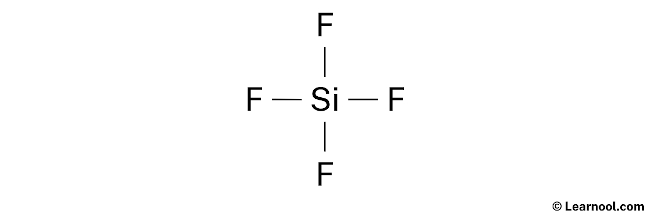
- Third, determine the central atom
We take to identify the least electronegative atom at the center.
Since silicon is less electronegative than fluorine, presume that the cardinal atom is silicon.
Therefore, place silicon in the center and fluorines on either side.
- And finally, describe the rough sketch
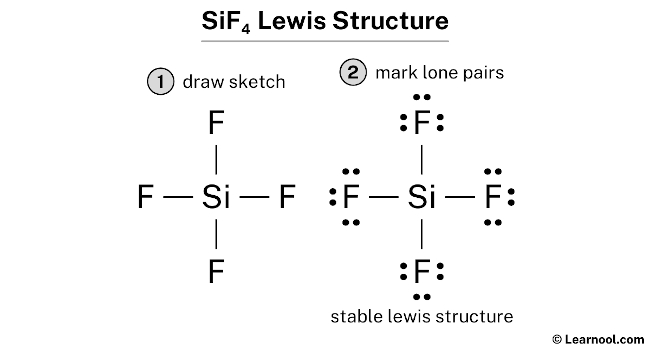
#2 Marker Lone Pairs
Here, we accept a full of sixteen electron pairs. And four Si — F bonds are already marked. So we take to only mark the remaining twelve electron pairs as lone pairs on the sketch.
As well recollect that silicon is a period iii element, and so it can continue more than 8 electrons in its terminal shell. And fluorine is a period 2 element, so it can not continue more than 8 electrons in its final beat out.
Always offset to mark the lone pairs from outside atoms. Here, the outside atoms are fluorines.
And so for each fluorine, there are three lone pairs, and for silicon, at that place is zippo lone pair considering all twelve electron pairs are over.
Mark the alone pairs on the sketch as follows:
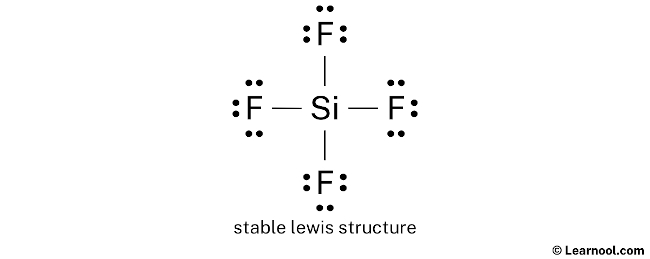
#3 Mark Charges
Use the following formula to calculate the formal charges on atoms:
Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons
For silicon cantlet, formal charge = 4 – 0 – ½ (viii) = 0
For each fluorine cantlet, formal charge = 7 – vi – ½ (2) = 0
Here, both silicon and fluorine atoms do not take charges, so no need to mark the charges.
In the in a higher place structure, you can see that the primal cantlet (silicon) forms an octet. And the outside atoms (fluorines) too class an octet. Hence, the octet rule is satisfied.
Therefore, this construction is the stable lewis structure of SiF4.
Next: CHthreeF Lewis Structure
Sif4 Lewis Structure Molecular Geometry,
Source: https://learnool.com/sif4-lewis-structure/
Posted by: randolphhavall.blogspot.com


0 Response to "Sif4 Lewis Structure Molecular Geometry"
Post a Comment